Dr. Deniz Dalkara, tenured researcher in “Gene therapies and animal models for neurodegenerative diseases” lab, INSERM, CNRS, Sorbonne Université, at Institut de la Vision, gives us the secrets of her research work currently in progress in the laboratory on inherited retinal degenerations. This project received financial support from the DIM Gene Therapy and the Paris Region.
Why treatment development for inherited retinal degenerations is such an important matter?
D. D: Diseases like macular degeneration, affect 30 million elderly people worldwide whereas inherited retinal degenerations like retinitis pigmentosa (RP) cause 1.5 million working age people to become legally blind by the age of 40. Vision loss is a feared handicap. As treatment options for photoreceptor degenerative diseases are currently severely limited, there is an urgent need for the development of new therapies. This has motivated during the past decade, significant progress in our understanding of molecular and genetic mechanisms of inherited retinal degenerations. Armed with this knowledge, researchers have now the opportunity to develop many new therapies using cutting-edge biotechnologies such as gene therapy, cell therapy and optogenetics for a large number of retinal diseases.
In your laboratory, are you working on the application of these new biotechnologies?
D. D: In my lab, we investigate various approaches of gene delivery to develop effective gene-based therapies for retinal degenerative disease and create animal models of human diseases that accurately mimic the molecular mechanisms of disease. In a normal retina, photoreceptors are the primary light-sensing cells which develop connections with other cells for transforming light into biological signals, transmitted through the optic nerve to the brain for visual recognition. In patients who have lost their photoreceptors, signal transmission and light sensitivity are not possible anymore. The work we published recently (Garita-Hernandez et al., Nature Comms 2019), combines the power of several new biotechnologies such as optogenetics in synergy with cellular therapy to help patients who have lost their photoreceptors to recover vision. Cell therapy offered the possibility of replacing the dying photoreceptors with new ones. In the lab, we are able to generate high numbers of replacement photoreceptors from human induced pluripotent stem cells (adult cells that have been genetically reprogrammed to an embryonic stem cell–like state) and in their differentiated forms; these cells display many features of photoreceptor cell identity. The catch is that they have a very difficult time forming the outer segments, that part that makes them sensitive to light and crucial for the signal transmission to the brain. Moreover, even if such structures do form, they still need to be regenerated continuously for phototransduction to occur. This brings in an additional layer of complexity, requiring interplay between non-neuronal cells and the photoreceptors.
Needless to say, without such light capturing antenna (outer segment), there is little utility to grafting new photoreceptors into a blind subject’s retina. For a cell therapy approach that is independent from the formation and maintenance of the outer segments like in late stage retinal degeneration, we turned to optogenetics. This form of gene therapy uses microbial opsins (Light Sensitive Proteins from algae) delivered by viral vectors, into photoreceptors from human induced pluripotent stem cells that render cells sensitive to light. After transplantation into blind mice, we observed light-driven responses at the retinal and behavioural levels originating from graft. Light responses we recorded were characteristic of the inserted microbial opsins’ properties. These results demonstrate that structural and functional photoreceptor repair is possible by combining both stem cell therapy and optogenetics.
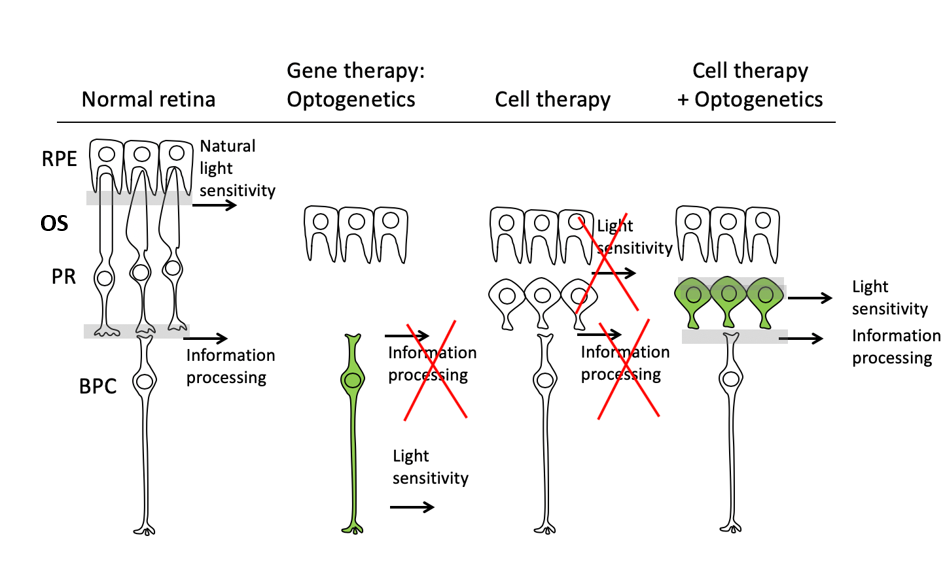
Figure Legend: Schematic representation of the three-fold challenge in photoreceptor cell replacement. In order to provide visual improvement, the transplanted photoreceptors need to form functional outer segments (OS), maintain light sensitivity, and develop connections with the host bipolar cells (BPC) for signal transmission. Optogenetics restores light sensitivity in the second or third order neurons of the retina but loses information processing of the inner retina. Transplanted photoreceptors fail to develop normal OS structure and lack light sensitivity. Introduction of a hyperpolarizing microbial opsin into the photoreceptor derivatives before transplantation provides a novel approach for vision restoration in late stage retinal degeneration. RPE- retinal pigment epithelium; PR- photoreceptor; BPC- Bipolar cells.
Why do you need to combine several technologies?
D. D: Because it is such a complex problem that we have tried to solve with new and emerging technologies which themselves have several limitations as of today. Thankfully each limitation can be compensated by the versatility of the other technology in our context. The synergy between the different approaches we used in this study also initiated a great collaboration between the authors of the study who come from different horizons: the background in optics, electrophysiology and retinal circuitry of Dr Jens Duebel, from Department of visual information processing at Institut de la Vision, was complemented by my expertise in gene delivery and therapy. Professor Marius Ader, group leader at CRTD/DFG-Center for Regenerative Therapies Dresden, University of Technology Dresden, Germany, and Dr Olivier Goureau, research director of “Retina development and regeneration” team at Institut de la Vision, brought in their knowledge of stem cell biology and transplantation.
What are the next steps of this project? Can we hope for a treatment soon?
D. D: The proof of concept of the synergy between cell therapy and optogenetics has been established in mice in our laboratory. Now, we wish to undertake the next steps of this strategy by transitioning to pre-clinical testing in non-human primates, an essential step before this therapy can be applied in patients. We have received funding from the America National Institute of Health under the Audacious Goals initiative to achieve the first steps towards this ambitious goal and are excited to move it forward towards clinical application in the years to come.

